Agricultural Literacy Curriculum Matrix
Lesson Plan
In Search of Essential Nutrients (Grades 9-12)
Grade Level
Purpose
Students explore the meaning of essential elements and use periodic tables to compare the elements that are essential to people and plants. Students discover where in the environment plants obtain each of their essential elements. Grades 9-12
Estimated Time
Materials Needed
Engage:
Activity 1: Essential Elements
- In Search of Essential Nutrients PowerPoint
- The Periodic Table, 1 per student
- 3 colored pencils or highlighters per student
Activity 2: Sources of Essential Elements
- In Search of Essential Nutrients PowerPoint (continued from Activity 1)
- Sources of Essential Elements, 1 per student
- Using Nitrogen, 1 per student
Activity 3: Humans, Food, and Essential Elements
- Food Label handout, 1 per student printed front to back
Vocabulary
macronutrient: a nutrient that must be present in a relatively large amount to ensure the health of the organism; building blocks used to make essential biomolecules
micronutrient: a nutrient required in small quantities to ensure the health of the organism; often used as cofactors for enzymatic reactions
micronutrient: a nutrient required in small quantities to ensure the health of the organism; often used as cofactors for enzymatic reactions
nutrient: a substance that provides nourishment essential for growth and the maintenance of life
Did You Know?
- Plants require 17 essential elements to complete their life cycle.
- Plants and humans require similar sets of essential elements.
- Plants obtain their essential elements from air, water, and soil.
Background Agricultural Connections
Plants and Their Essential Elements
All organisms must take in matter from their environment in order to survive. There are 92 naturally occurring elements on Earth. Living things need only a minority of them. For example, humans require about 21 different elements to be healthy. Almost all of the mass of our bodies comes from just six of those elements (carbon, hydrogen, oxygen, nitrogen, phosphorus , and calcium). These are the elements used to construct the carbohydrates, nucleic acids, proteins, and other molecules that make up our cells and carry out their chemistry. Other elements critical to our health are needed in very small amounts. Often, such elements are cofactors required by enzymes to catalyze specific chemical reactions. Regardless of whether elements are needed in large or small amounts, they must be obtained from the environment. Furthermore, it not enough that essential elements are present in the environment; they must be available in a chemical form that our bodies can use.
Not surprisingly, the situation in plants is similar. They, too, must carry out thousands of different chemical reactions, many of which are similar to those of humans. Scientists have identified 17 elements that are essential for plants (see the table below). An element is described as being essential to the plant if the following conditions are met:
- The element must be required by the plant to complete its life cycle.
- The element cannot be replaced by another element.
- The element must be required for a specific biological function.
- The element must be required by a substantial number of different plant species.
Essential elements can be classified as mineral or non-mineral nutrients. Carbon, hydrogen, and oxygen are classified as non-mineral nutrients because they are obtained from the atmosphere and water. Mineral nutrients can be further classified as being either macronutrients or micronutrients. As the name implies, macronutrients are needed in relatively large amounts. Nitrogen, phosphorous, and potassium are called primary macronutrients, while calcium, sulfur, and magnesium are called secondary macronutrients. The rest of the essential elements are called micronutrients because they are needed in small amounts. It is important to note that despite their name, micronutrients are just as essential to plant health as are macronutrients.
Plants absorb most of their essential elements from water in the soil. Usually the essential elements are taken up as a positively charged cation or a negatively charged anion.
| Element | Symbol | Classification | Chemical Form Taken into the Plant |
|---|---|---|---|
| Hydrogen | H | Nonmineral nutrient | H2O |
| Oxygen | O | Nonmineral nutrient | O2 and CO2 |
| Carbon | C | Nonmineral nutrient | CO2 |
| Nitrogen | N | Primary macronutrient | NH4+ and NO3- |
| Phosphorus | P | Primary macronutrient | H2PO4- and H2PO42- |
| Potassium | K | Primary macronutrient | K+ |
| Calcium | Ca | Secondary macronutrient | Ca2+ |
| Magnesium | Mg | Secondary macronutrient | Mg2+ |
| Sulfur | S | Secondary macronutrient | SO42- |
| Boron | B | Micronutrient | B(OH)3 |
| Chlorine | Cl | Micronutrient | Cl- |
| Copper | Cu | Micronutrient | Cu2+ |
| Iron | Fe | Micronutrient | Fe2+ and Fe3+ |
| Manganese | Mn | Micronutrient | Mn2+ |
| Molybdenum | Mo | Micronutrient | MoO42- |
| Nickel | Ni | Micronutrient | Ni2+ |
| Zinc | Zn | Micronutrient | Zn2+ |
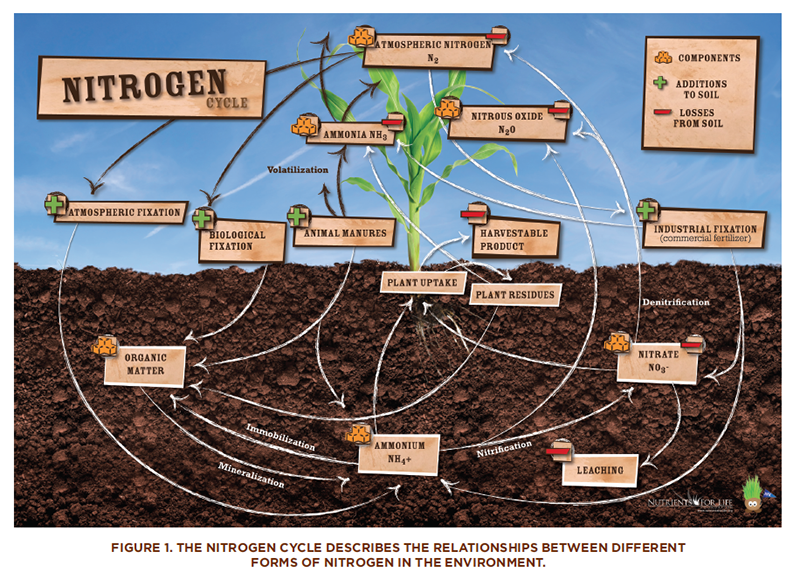
The Nitrogen Cycle
Although the atmosphere is about 78 percent nitrogen, plants cannot make use of nitrogen gas (N2). Instead, plants need to obtain their nitrogen by taking up the cation ammonium (NH4+) or the anion nitrate (NO3–) in the soil. These ionic forms of nitrogen are generated by the breakdown of organic material in the soil or through a process called nitrogen fixation that is carried out by soil microbes. Some crop plants (legumes such as peas, beans, peanuts, and soybeans) live in close association with nitrogen-fixing bacteria that live in their roots and convert N2 gas to a form that plants can use. Such crops have a steady source of nitrogen and do not require nitrogen-containing fertilizers.
The nitrogen cycle describes the processes by which nitrogen moves between its various chemical forms. Biological or physical processes can cause these chemical conversions. Four processes are essential to the nitrogen cycle.
- Nitrogen fixation refers to the process by which atmospheric nitrogen (N2) is converted to nitrogen–containing compounds that are usable by plants. Nitrogen fixation can be accomplished through the action of lightning or bacteria in the soil.
- Ammonification refers to the process by which bacteria and fungi convert decomposed nitrogen-containing compounds into ammonium ions (NH4+).
- Nitrification refers to the process by which bacteria convert ammonium ions into nitrite (NO2-). Other bacteria convert nitrite to nitrate (NO3-). This is important because nitrites can reach levels that are toxic to plants.
- Denitrification refers to the process by which bacteria convert nitrates back to N2.
So, let us summarize the nitrogen cycle. First, recall that plants cannot use the nitrogen in the air that is so plentiful. When plants and animals die and decompose, they add nitrogen to the soil. Bacteria in the soil convert the nitrogen into compounds that plants can use. Plants take in these nitrogen-containing compounds through their roots and use them to grow. Animals eat the plants, use the nitrogen, and return it to the soil when they die and decompose.
Engage

- Project the In Search of Essential Nutrients PowerPoint. Explain that scientists who are interested in studying human health must understand the specific needs of the body. Ask students, “What do humans need to live?” (slide 2) Record student responses on the board. Accept all answers. Students will likely recognize air, water, food, sleep, and environmental conditions such as temperature and pressure, or material items such as clothing and shelter.
- Referring to the list generated from step one, ask students, “Which of these items come from the environment?” Students should recognize that most (or all) of the items necessary for survival are obtained from the environment.
- Direct the discussion to focus on air (oxygen), water, and food (slide 3). Ask students, “Why do we need each of these (air, water, and food) to survive?” Students should recognize that:
- Oxygen in the air is needed for cellular respiration.
- Our cells are mostly made of water and water is the medium in which life has evolved. It is required for the chemistry of life.
- Food has two critical functions as a source of chemical energy and as a source of chemical building blocks needed by our cells.
- Remind students that humans (and animals) eat plants and other animals to obtain chemical energy and provide them with the building blocks needed by their cells. Ask students, “What about plants; do plants need food?” (slide 4) Some students may respond that plants do not need food, because they can obtain energy from photosynthesis. Other students may mention that plants need water or that they obtain nutrients from the soil. If not mentioned by a student, remind the class that fertilizer can be considered “food” for plants, because it provides nutrients that plants need to live and grow.
| In many things you may read online, the terms “essential elements” and “essential nutrients” are often used interchangeably. A nutrient is a substance that organisms need to live and grow. In this activity, the term essential elements will be the preferred term because students will be looking at the periodic table of elements. Elements often combine into larger molecules that living things use. For example, water is an important nutrient for organisms; water is made up of the elements hydrogen and oxygen. |
Explore and Explain
Activity 1: Essential Elements
In this activity, students use the periodic table to express their prior knowledge about what plants need to survive. Their predictions are compared to a list of essential elements known to be important to plant health.
- Explain that they will now investigate the chemical elements that are essential for plant growth.
- Project slide 4 of the PowerPoint titled, An Essential Element. Ask different students to read aloud the criteria that describe an essential element.
- Pass out to each student one copy of The Periodic Table. Instruct the class to think about the definition of “essential element.”
- Project The Periodic Table (slide 6) on your white board, ask for a student volunteer to highlight or outline the elements that are essential for healthy plant growth. If possible, students should think of an example of how a given element is used by the plant (such as the plant using nitrogen to make protein or phosphorus being used to make ATP).
- Students likely will not be able to suggest a function for elements needed in trace amounts. Many such elements are needed as cofactors for enzymes. It is not important to discuss the uses of each element, but it is important that students understand that these elements are needed to build cell structures and to carry out the cell’s chemistry through enzymatic reactions. This step gives you an opportunity to assess how well students can relate their knowledge of chemistry to biology. For example, students may respond that carbon is used to make carbohydrates, such as sugar.
- Have additional students add to the list with their predictions and explanations.
- Explain that you are now going to reveal which elements have been shown to be essential for plant growth and compare them with their predictions. Project slide 7, Essential Elements for Plants.
- Students likely will be surprised that so many elements are essential for plant growth. The comparison between the elements predicted by the students and the accepted ones should show some overlap. Students should be expected to identify carbon(C), hydrogen(H), nitrogen(N), oxygen(O), phosphorus(P), and sulfur(S) because these elements serve as building blocks for biomolecules. If necessary, ask guiding questions to connect these elements to the synthesis of proteins, nucleic acids, and carbohydrates.
- Ask, “Do you think that humans require the same essential elements as plants?” Responses will vary. Some students may think that since humans and plants are very different from each other, they will need different sets of elements. Others may reason that since plants and humans are each made of cells that contain similar biomolecules, the essential elements needed by both will be similar.
- Project slide 8, Essential Elements for Humans. Ask students to observe how similar or dissimilar this pattern of elements is compared with that shown previously for plants. Continue to slide 9 and ask the following questions:
- What is similar?
- What is different?
- Are the needs of humans and plants more alike or more different? (similar)
- Instruct students to use 3 colored pencils or highlighters and color code their Periodic Table handout indicating which elements are required by humans, which are required by plants, and which are required by both plants and animals.
Activity 2: Sources of Essential Elements
In this activity, students consider the source of essential elements for plants.
- Explain that you will conclude the lesson with a brief activity that explores where plants obtain their essential elements.
- Pass out to each student one copy of the handout, Sources of Essential Elements. Explain that the handout lists the 17 essential plant elements. Instruct students to think about where a plant obtains these essential elements. Students should indicate the source—air, water, and soil—for each element (that is, each chemical element) by checking the appropriate boxes on the handout.
- For the purpose of this activity, students should think about water as rainfall (before it reaches the ground). It therefore should not include those elements found in soil that may dissolve in water. Students are free to check more than one box for any element. Give students about 5 minutes to complete this task.
- Project slide 10, Sources of Essential Elements on the board. Ask a student volunteer to describe which elements he or she listed as coming from water.
- Put a “W” next to the elements named by the students. Of course, students should mention hydrogen and oxygen. Actually, rainwater may contain small amounts of other elements derived from atmospheric gases and dust particles. Other elements that could be mentioned include C, Cl, N, and S.
- Ask another volunteer to describe which elements he or she listed as coming from the air.
- Put an “A” next to the elements named by the students. Students should recognize that the corn plant obtains carbon and oxygen (via CO2) from the air. Some students may know that most of the atmosphere is nitrogen. Most students will not realize that nitrogen gas is not available to the plant in a usable form. Do not correct this misconception yet. This issue will be addressed later. As with water, small amounts of other elements may also be present due to air pollution.
- Ask another volunteer to describe which elements he or she listed as coming from the soil.
- Put an “S” next to the elements named by the students. Students should list most if not all of the essential elements. The soil not only contains many elements that reflect its geological history, but it also contains organic material from once-living plants and animals as well as from the abundant life (both macro and micro) that resides there.
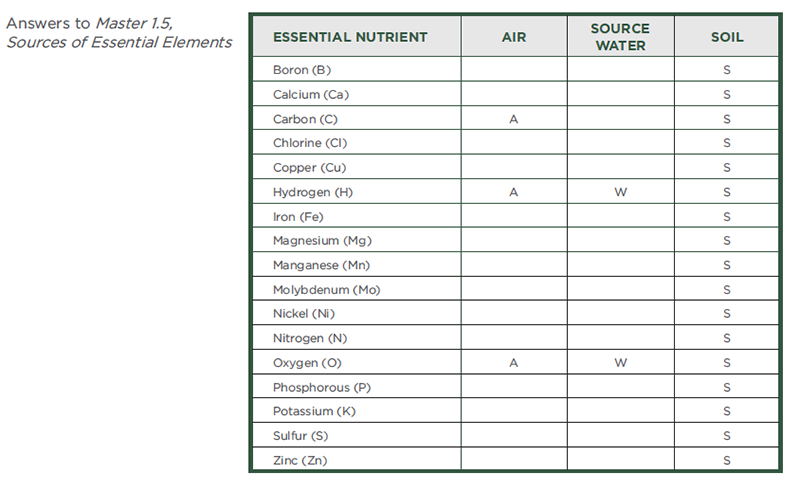
- Put an “S” next to the elements named by the students. Students should list most if not all of the essential elements. The soil not only contains many elements that reflect its geological history, but it also contains organic material from once-living plants and animals as well as from the abundant life (both macro and micro) that resides there.
- Ask students to help you summarize where plants get their essential elements. Students should report the following (slide 11):
- Water: Hydrogen and oxygen.
- Air: Carbon and oxygen.
- Soil: All essential elements.
- Ask students to work individually or in pairs to write a short summary before holding a class discussion. This will allow students to gather their thoughts before speaking and for you to assess each student’s understanding.
- Explain that nitrogen is an essential element that plants need in relatively large amounts. Pass out to each student 1 copy of the Using Nitrogen handout. Instruct students to read the description and answer the questions.
- After students have completed their tasks, ask them, “In light of what you just read, would you change your prediction of where plants obtain nitrogen?” (slide 12). Students should change their answer, if necessary, to indicate that plants must obtain nitrogen from the soil rather than from the air (slide 13).
- Ask for volunteers to read their answers to Question 1 and 2 on the Using Nitrogen handout.
- Question 1: What do you think is responsible for converting most of the nitrogen used by plants into a usable form?
- Answer: Students should conclude that bacteria are responsible for fixing most of the nitrogen used by plants. Some nitrogen is also fixed by lightning and industrial processes, but these are much smaller amounts (slide 13).
- Question 2: Why is this ability of legumes to carry out their own nitrogen fixation important to farmers?
- Answer: Because the symbiotic bacteria in legumes fix additional nitrogen for plants to use, farmers can be less concerned with replenishing the soil by using nitrogen-containing fertilizers.
- Question 1: What do you think is responsible for converting most of the nitrogen used by plants into a usable form?
- Explain that to grow healthy crops, farmers need to know which essential elements are found in the soil and how much of each is present. Ask students to think of where the essential elements found in the soil come from. Student responses will vary. Focus on the following:
- natural sources, such as the erosion of rocks;
- the action of lightning;
- the decomposition of plant and animal material;
- human-associated activities, such as runoff from fertilizers used by farmers and the public as well as from waste that humans produce; and
- emissions from industry and automobiles.
Activity 3: Humans, Food, and Essential Elements
This activity requires a variety of different food labels and access to the Internet if carried out in the classroom. Alternatively, it can be assigned as homework.
- Ask students to recall that plants get their essential elements mostly from the soil. Ask, “What about people? Where do they get their essential elements?
- Students should respond that people get most of their essential elements from food, though water gives us hydrogen and oxygen just as it does for plants.
- Instruct students to obtain a food label from a nutritious food for analysis. Since this activity is concerned with diet and essential elements needed for good heath, food labels should come from healthy foods and not from snacks.
- Give each student one copy of the Food Label handout and instruct them to follow the directions.
- Some elements, including sodium, calcium, and magnesium are listed on food labels. However, most of the ingredients listed on a food label are chemical compounds and not individual elements.
- After students have completed their task, ask volunteers to list the essential elements found on their food labels. List them on the board as well as the chemical compound they come from.
- Ask if other students found any additional essential elements to add to the list. As before, list the ingredient in which each essential element is found.
- Ask students to compare the list of essential elements from their food labels to those shaded as essential on the periodic table on the back of their handout. Students will see that the nutrition facts label on foods includes data about elements, such as calcium, iron, zinc, or manganese content. Students will see that food ingredients are the source of the 6 elements (C, H, N, O, P, and S) that are needed to make important biomolecules when they research the molecular formula. For example, they will see that carbohydrates and fats are made up of carbon, hydrogen, and oxygen. Proteins also contain nitrogen, phosphorus, and sulfur. Many of the other essential elements that are not structural components of biomolecules are needed as cofactors for enzymes and are present in very small amounts.
- Conclude the activity by asking, “What did this exercise teach you about health and diet?” Students should recognize that their diet needs to contain a variety of foods to supply all the essential elements. Plants, as well as people, need a “balanced diet.”
|
Three Dimensional Learning Proficiency: Disciplinary Core Ideas
Three Dimensional Learning Proficiency: Crosscutting Concepts Students link different domains of science fields into a coherent and scientifically-based view of the world.
|

Elaborate
This lesson is the first in a series of five related lessons. Refer to the following lessons for further depth:
- Lesson 1: In Search of Essential Nutrients
- Lesson 2: Properties of Soils
- Lesson 3: Plant-Soil Interactions
- Lesson 4: Plant Nutrition Deficiencies
- Lesson 5: Fertilizers and the Environment
Evaluate
After conducting these activities review and summarize the following key concepts:
- Humans and plants need essential elements to live.
- For plants, the majority of their essential elements are found in the soil.
- To grow healthy crops, farmers need to know which essential elements are found in the soil and how much of each is present.
Sources
- Nutrients for Life Foundation
- BSCS-Biological Science Curriculum Study
- Reviewed by Smithsonian Institution
Recommended Companion Resources
Author
Organization
| We welcome your feedback! If you have a question about this lesson or would like to report a broken link, please send us an email. If you have used this lesson and are willing to share your experience, we will provide you with a coupon code for 10% off your next purchase at AgClassroomStore. |
